Abstract
A major problem of current vaccines is storage stability, often requiring strict maintenance of cold chains. In the course of the eradication of smallpox, a freeze-dried vaccinia virus (Dryvax), which proved to be very stable, was used to overcome this limitation. However, Dryvax needs to be reconstituted before usage and is administered using a bifurcated needle, procedures that pose a number of additional health risks. We report in this study that a stable, lyophilized, modified vaccinia virus Ankara (MVA) vaccine can be directly applied to the nostrils of mice without previous reconstitution. This direct mucosal application induced systemic Ab and T cell responses comparable to those achieved by i.m. administration. Importantly, mucosal application of lyophilized MVA induced long-lasting protective immunity against lethal bacterial and viral challenges. These data clearly demonstrate the potency of a simple needle-free vaccination, combining the advantages of mucosal application with the stability and efficiency of lyophilized MVA.
Preventative vaccination has proven to be extremely successful against several threatening infectious diseases, including smallpox. Yet, the current means of applying vaccines are not satisfactory. For example, most vaccines still require the use of needles. The pain associated with and anticipated for needle injections is a source of great anxiety and distress, not only for children but also for adults, and severely affects the broader acceptance of conventional vaccines. Even more important, each year an overwhelming number of infections with HIV (80,000–160,000), hepatitis C virus (2.3–4.7 million), and hepatitis B virus (8–16 million) are thought to originate from the reuse of needles and syringes by health care providers (1). The need to develop alternative vaccination strategies to deliver vaccines has resulted in several new techniques (reviewed in Refs. 2, 3). These techniques target either the skin or mucosal tissue to induce systemic and/or mucosal immune responses.
Successful vaccination strategies should be effective, relatively simple to administer, and cost efficient. Furthermore, to fully control or even eradicate an infectious disease, vaccines must be stable to be broadly distributed, which is often a difficult task in developing countries. In addition, long-term storage is a strong requirement to cover unpredictable new outbreak situations. In the course of the smallpox eradication campaign, this important step was achieved by lyophilization of the vaccinia virus (VACV)3 Dryvax. Dryvax remains stable under this condition even in tropical countries, thus avoiding the dependency on cold chains, but it is reconstituted and administered using a bifurcated needle.
The next generation smallpox vaccine and viral vector system being already used in clinical studies is modified VACV Ankara (MVA). Its use for primary smallpox vaccination in > 100,000 humans proved to be entirely unproblematic. Therefore, clinical studies using MVA as a viral vector system against HIV and tumors have been conducted (4, 5). Additionally, several studies have demonstrated the ability of MVA to induce robust immune responses when applied by mucosal routes (6), which was recently confirmed in nonhuman primates by aerosol administration (7).
In an effort to combine the advantageous properties of MVA with the most simple and feasible approach of needle-free administration, we decided to evaluate the efficacy of direct nasal application of lyophilized MVA. Particularly, we were interested in determining the induction of systemic cellular and humoral immune responses and the potential of the vaccine to confer long-term protective immunity.
Materials and Methods
Mice and vaccination
C57BL/6 mice were derived from in-house breeding under specific pathogen-free conditions following institutional guidelines or from Taconic. Only female mice between 8 and 12 wk of age were used. Mice were vaccinated with 108 infectious units of MVA i.m. or with different doses of lyophilized MVA. For mucosal application, MVA was freeze dried in 100 μl of Tris buffer in a 2-ml Eppendorf tube. The resulting lyophilizate was cut into four pieces, picked up with a small wire, and applied onto the nostrils of anesthetized mice where it quickly dissolved into the nasal cavity.
Viruses
The VACV strain Western Reserve (WR) was provided by B. Moss (National Institutes of Health, Bethesda, MD). MVA (cloned isolate IInew) expressing the entire OVA gene under the control of the modified PH5 promoter was generated as described previously (8).
Quantification of Ag-specific T cell responses
Splenocytes from vaccinated C57BL/6 mice were stimulated with either H-2Kb- or H-2Db-restricted VACV-specific peptides derived from A3L270, A8R189, B8R20, K3L6, OVA257, or a control peptide (β-galactosidase96) for 5 h in the presence of 1 mg/ml brefeldin A (Sigma-Aldrich). For evaluation of CD4 T cell responses, splenocytes were incubated with L4R176; for OVA-specific CD4 T cell responses, splenocytes were incubated with100 μg/ml OVA (Sigma-Aldrich) for 2 h before the addition of brefeldin A. Cells were live/dead-stained with ethidium monoazide bromide (Invitrogen) and blocked with anti-CD16/CD32-Fc-Block (BD Biosciences). Surface markers were stained with anti-CD8 (5H10; Caltag Laboratories), anti-CD62L (MEL-14), and anti-CD127 (A7R34; all from eBioscience). Intracellular cytokine staining was performed with anti-IFN-γ (XMG1.2), anti-TNF-α (MP6-XT22; both from BD Biosciences), and anti-IL-2 (JES6-5H4; eBioscience) using the Cytofix/Cytoperm kit (BD Biosciences). Data were acquired by FACS analysis on a FACSCanto flow cytometer (BD Biosciences) and analyzed with FlowJo software (Tree Star).
Viral and bacterial challenges
Fifty days after immunization, animals were infected intranasally (i.n.) with 106 PFU of VACV WR diluted in 30 μl of PBS and monitored for 3 wk with daily measurement of individual body weights. Animals suffering from severe systemic infection and having lost 30% of body weight were killed. The mean change in body weight was calculated as the percentage of the mean weight for each group on the day of challenge. To evaluate Listeria monocytogenes (L.m.)-OVA-specific protection, animals were infected i.v. with 2.5 × 105 CFU of L.m.-OVA. Three days later, spleen and liver were harvested and the bacterial load was analyzed by plating out serial dilutions.
ELISA
Maxisorp plates (Nunc) were coated with sucrose gradient-purified MVA (at a protein concentration of 1 μg/ml) or 100 μg/ml OVA (Sigma-Aldrich) overnight and blocked for 60 min. Serial dilutions of serum or fecal pellet samples were incubated and measured at 405 nm.
Statistical analysis
All statistical analysis was performed using Excel software. Results are expressed as the mean of the SD. Differences between groups were analyzed for statistical significance using a two-tailed Student t test.
Results
To analyze the efficacy of nasally applied lyophilized MVA, we performed a dose titration. Different amounts of MVA (infectious units) were lyophilized in 100 μl of buffer. The resulting lyophilizate was split into equal aliquots and subsequently applied into the nostrils of anesthetized mice where it quickly dissolved. Eight days later, we analyzed Ag-specific T cell responses in the spleen using MHC multimers and intracellular cytokine staining. We found a dose-dependent linear correlation between the amounts of applied MVA and the resulting T cell response (Fig. 1). CD8+ T cell responses against the dominant determinant B8R20 ranged from ∼1 to 9% by MHC multimer staining for detection using increasing amounts of MVA ranging from 106 to 109 infectious units. Ag-specific T cells were functional and ranged from 0.3 to ∼7% when analyzing intracellular IFN-γ production. We also detected robust immune responses against the subdominant determinants A3L270, A8R189, and K3L6 after nasal immunization with 109 infectious units (Fig. 1 C), with a similar immunodominance hierarchy as that reported for i.p. administration (9, 10). We concluded that 109 infectious units is a sufficient dose for the induction of a broad cellular immune response.
Lyophilized MVA applied mucosally elicits dose-dependent immune responses. A and B, Groups of mice (n = 4) were vaccinated i.n. with different doses of lyophilized MVA wt. On day 8, splenocytes were analyzed for multimer binding against the immunodominant epitope B8R20 (A) or for intracellular IFN-γ production (B). Numbers in the plots represent the mean proportion of B8R20-specfic CD8+ cells ± SD. C, Analysis of one representative experiment of three independent experiments. Tet, Tetramer.
Lyophilized MVA applied mucosally elicits dose-dependent immune responses. A and B, Groups of mice (n = 4) were vaccinated i.n. with different doses of lyophilized MVA wt. On day 8, splenocytes were analyzed for multimer binding against the immunodominant epitope B8R20 (A) or for intracellular IFN-γ production (B). Numbers in the plots represent the mean proportion of B8R20-specfic CD8+ cells ± SD. C, Analysis of one representative experiment of three independent experiments. Tet, Tetramer.
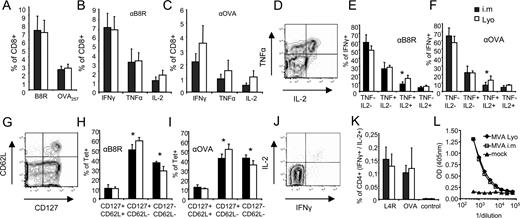
Next we examined the induction of T cells directed against a recombinant model Ag (OVA) using 109 infectious units of lyophilized OVA-expressing recombinant MVA (MVA-OVA), we compared the immunogenicity of this route of immunization to the standard i.m. immunization using 108 infectious units of the same virus. Notably, i.m. administration of an increased dosage of MVA (109infectious units) did not yield significantly higher T cell responses than the standard dose (108 infectious units) (supplemental Fig. S1).4 When comparing both vaccination routes, we found similar immune responses against B8R20 and OVA257 as determined by MHC multimer binding (Fig. 2,A). Additionally, CD8+ T cells induced by either route produced comparable amounts of IFN-γ, TNF-α, and IL-2 (Fig. 2, B and C).
Mucosally applied lyophilized MVA OVA compares to i.m. application. A–C, Groups of mice (n = 4) were vaccinated i.n. with lyophilized MVA OVA (109 infectious units; □) or i.m. (108 infectious units; ▪). On day 8 postpriming, splenocytes were analyzed for multimer binding against the immunodominant VACV epitope B8R20 or OVA257 (A) or for intracellular IFN-γ, TNF-α, or IL-2 production against VACV- B8R20 (B) or OVA257 (C). D, IFN-γ positive cells were further analyzed for multifunctionality. E and F, Distribution of multifunctional subpopulations after stimulation with VACV- B8R20 (E) or OVA257 (F). G, Multimer positive cells were further analyzed for CD62L and CD127 subpopulations. H and I, Distribution of memory T cell subpopulations of VACV- B8R20 (H) or OVA257 (I). J, CD4+ T cells were analyzed for IL-2 and IFNγ production after peptide/protein stimulation. K, Similar cytokine production of CD4+ T cells for both routes against VACV-L4R176–190 or OVA protein. L, ELISA of VACV-specific serum IgG for both routes (day 45). All data are representative for three independent experiments. (∗, p < 0.05). αB8R, Anti-B8R; αOVA, anti-OVA.
Mucosally applied lyophilized MVA OVA compares to i.m. application. A–C, Groups of mice (n = 4) were vaccinated i.n. with lyophilized MVA OVA (109 infectious units; □) or i.m. (108 infectious units; ▪). On day 8 postpriming, splenocytes were analyzed for multimer binding against the immunodominant VACV epitope B8R20 or OVA257 (A) or for intracellular IFN-γ, TNF-α, or IL-2 production against VACV- B8R20 (B) or OVA257 (C). D, IFN-γ positive cells were further analyzed for multifunctionality. E and F, Distribution of multifunctional subpopulations after stimulation with VACV- B8R20 (E) or OVA257 (F). G, Multimer positive cells were further analyzed for CD62L and CD127 subpopulations. H and I, Distribution of memory T cell subpopulations of VACV- B8R20 (H) or OVA257 (I). J, CD4+ T cells were analyzed for IL-2 and IFNγ production after peptide/protein stimulation. K, Similar cytokine production of CD4+ T cells for both routes against VACV-L4R176–190 or OVA protein. L, ELISA of VACV-specific serum IgG for both routes (day 45). All data are representative for three independent experiments. (∗, p < 0.05). αB8R, Anti-B8R; αOVA, anti-OVA.
Recent studies have demonstrated the importance of multifunctional T cells for protection against pathogens (reviewed in Ref. 11). Therefore, we additionally analyzed the relative distribution of multifunctional T cells induced via i.n. or i.m. routes. Fig. 2,D shows representative staining of TNF-α and IL-2 gated on IFN-γ+ cells upon previous stimulation with B8R20 or OVA257 peptide. We found significantly more T cells producing all three cytokines when immunized i.n. with the lyophilized vaccine as compared with i.m. vaccination (Fig. 2, E and F). Based on the expression of CD62L and the IL-7 receptor (CD127) within MHC multimer binding T cells, we analyzed the distribution of T cell subpopulations (Fig. 2,G), which indicates the degree of differentiation into central memory, effector memory, and effector T cell populations (12, 13). The relative distribution of CD127+/CD62L+ (central memory) cells was similar in both routes. Yet, i.m. administration induced significantly less CD127+/CD62L− (effector memory), but significantly more CD127−/CD62L− (effector) cells as compared with i.n. administrated lyophilized MVA OVA. This different distribution was observed for both B8R20- and OVA257-specific T cells (Fig. 2, H and I). The trend toward a more differentiated phenotype of induced CD8+ T cells was even more pronounced when using higher doses of MVA i.m. (supplemental Fig S1).
The induction of Ag-specific CD4+ T cells has been suggested to be critical for the induction and maintenance of fully functional CD8+ memory T cells, as well as for the induction of germinal center reactions promoting Ig class switching and potent humoral immune responses (for review see Refs. 14 and 15). We found similar Ag-specific CD4+ T cell responses directed against the viral vector (L4R176) or against the target Ag OVA for both routes as detected by intracellular cytokine staining for IL-2 and IFN-γ (Fig. 2, J and K). In accordance with this observation, we found equal levels of IgG directed against MVA for both routes as measured by ELISA in the serum of mice 45 days postvaccination (Fig. 2 L). Additionally, mucosal administration of MVA induced significantly higher mucosal MVA-specific IgA levels as compared with i.m. administration (supplemental Fig. S2). Overall, we found similar systemic cellular and humoral immune responses when comparing lyophilized i.n. applied MVA to standard i.m. applied MVA.
A potential limitation for the use of viral vector-based vaccines is preexisting antivector immunity (16). Although Belyakov et al. have shown that mucosal vaccination with VACV can overcome this problem (17), we observed a similar impairment for the neoinduction of target Ag-specific T cell responses upon secondary vaccination by mucosal administration using lyophilized MVA or by the standard i.m. route (supplemental Fig. S3).
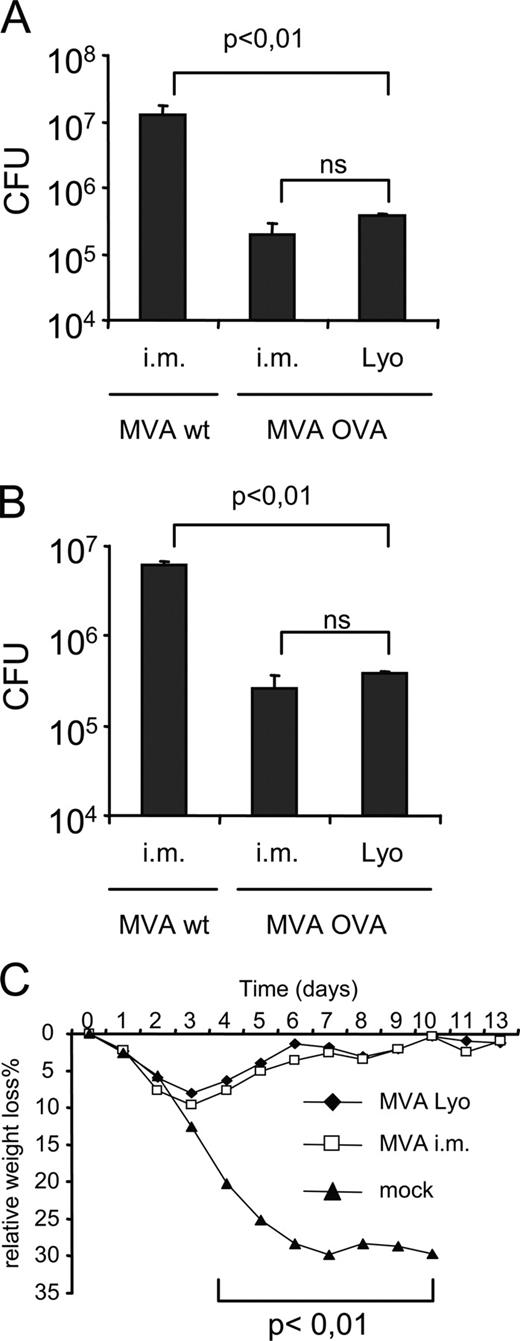
Next, we sought to determine the potency of both routes to confer protective immunity in the memory phase (day 50) postimmunization. As a model for bacterial infection we chose L.m. because protection against this intracellular pathogen is conferred almost exclusively by Ag-specific CD8+ T cells. We primed mice i.n. with lyophilized MVA OVA, i.m. with MVA OVA, or i.m. with MVA wild type (wt) as a control. Fifty days later, mice were challenged i.v. with L.m-OVA. Three days later, we analyzed the bacterial burden in the livers and spleens of the mice. With either immunization we found a reduction by >1 log of L.m.-OVA as compared with MVA wt-immunized mice (Fig. 3, A and B). This significant difference demonstrates the protective capacity of MVA vaccination by both routes. To analyze protection against an acute viral infection, we challenged vaccinated mice with VACV strain WR. In this model, protection is mainly conferred by neutralizing Abs (18). Mice were vaccinated i.n. with lyophilized MVA wt or i.m. with MVA wt or were mock vaccinated. Mock-immunized mice suffered from progressive disease and died between days 7 and 10 postchallenge (Fig. 3 C). In contrast, MVA-immunized mice had a maximum weight loss of ∼10% at day 3 postchallenge and then recovered, reaching their original weight at day 10 postchallenge.
Mucosally applied lyophilized MVA protects against lethal VACV and L.m.-OVA challenges. A and B, Groups of mice (n = 4) were primed i.m. with MVA wt or MVA OVA (108 infectious units) or mucosally with lyophilized MVA OVA (109 infectious units). On day 50 postpriming, mice were challenged with L.m.-OVA (2.5 × 105 CFU) i.v. MVA OVA-immunized mice showed a significant reduction of CFU as compared with MVA wt in liver (A) and spleen (B). For VACV challenge, mice were vaccinated i.m. (108 infectious units) or mucosally with lyophilized MVA wt (109 infectious units) or mock. On day 50 postpriming, mice were challenged with VACV (WR) i.n. with 106 PFU. C, Relative weight loss over time. Three of four mice from the mock-vaccinated group died on day 7, and one mouse died on day 10. Data are one representative of three independent experiments.
Mucosally applied lyophilized MVA protects against lethal VACV and L.m.-OVA challenges. A and B, Groups of mice (n = 4) were primed i.m. with MVA wt or MVA OVA (108 infectious units) or mucosally with lyophilized MVA OVA (109 infectious units). On day 50 postpriming, mice were challenged with L.m.-OVA (2.5 × 105 CFU) i.v. MVA OVA-immunized mice showed a significant reduction of CFU as compared with MVA wt in liver (A) and spleen (B). For VACV challenge, mice were vaccinated i.m. (108 infectious units) or mucosally with lyophilized MVA wt (109 infectious units) or mock. On day 50 postpriming, mice were challenged with VACV (WR) i.n. with 106 PFU. C, Relative weight loss over time. Three of four mice from the mock-vaccinated group died on day 7, and one mouse died on day 10. Data are one representative of three independent experiments.
Our data demonstrate that the direct nasal application of lyophilized MVA generated robust immune responses in a dose-dependent manner. The induced cellular and humoral immune responses were similar to those achieved by standard i.m. administration of the same viral vector. Mice receiving nasally applied lyophilized MVA were protected in the memory phase (day 50) against a lethal bacterial and viral challenge.
Discussion
To address the issue of needle-free administration of safe and efficient vaccines, we tested the capacity of lyophilized MVA to confer protective immunity by simply applying it nasally as a powder without reconstitution. Until now, other studies using lyophilized vaccines were unable to demonstrate the induction of a potent long-lasting and protective T cell immune response even when using live viruses (19, 20). In contrast, lyophilized MVA proved to be effective, although a slightly higher dose as compared with that for standard i.m. administration was required to achieve similar immunogenicity. The need for increased dosage could be due to the strong enzymatic activity (e.g., proteases) in the nasal tissue, which potentially inactivates the applied virus or impairs reconstitution of the lyophilizate in the nasal fluid. We were, to date, unable to quantify exactly how much of the lyophilized virus gets reactivated by nasal fluids. In vitro, 50–90% infectivity could be recovered after reconstitution (data not shown). In that respect, optimizing and standardizing the lyophilization process might improve the recovery rate. We hypothesize that the nasal administration as performed in this study is more localized and causes less inflammation as compared with standard i.m. vaccination. This could also explain why lyophilized MVA induced relatively more effector memory and less effector cells than i.m. applied MVA (21, 22). In line with this observation, relatively more polyfunctional (IFN-γ+/TNF-α+/IL-2+) T cells and less monofunctional (only IFN-γ+) T cells were induced by lyophilized MVA. This relative shift toward a more differentiated phenotype was even more enhanced when applying 109 infectious units i.m. (supplemental Fig. S1), in line with the role for inflammation and the amount of Ag on T cell differentiation as previously reviewed (11). Although we could not detect functional consequences concerning these subtle differences between both routes, one may anticipate that less inflammation could contribute fewer side effects of the vaccines when applied in humans. Translation of i.n. lyophilized vaccine application to humans seems simple, as the lyophilized virus-containing powder could just be “sniffed.” Immunizing mice by simply applying lyophilized MVA directly to the easily accessible nasal mucosa, we demonstrate the potential of a safe and clinically relevant vector vaccine to induce protective humoral and particularly cellular memory immune responses.
Acknowledgments
We thank R. Baier for excellent technical support and C. Fieder for excellent support in the mouse facility. We thank C. Koch for lyophilizing the virus and Scott N. Mueller for critically reading the manuscript. This work was supported by the DFG grant SFB 456 to I. D.
Disclosures
The authors have no financial conflict of interest.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Abbreviations used in this paper: VACV, vaccinia virus; i.n., intranasal(ly); L.m., Listeria monocytogenes; MVA, modified VACV Ankara; WR, Western Reserve.
The online version of this article contains supplemental material.